Vinyl acetate undergoes several important chemical reactions, the most significant being polymerization, hydrolysis, and transesterification.
- Polymerization
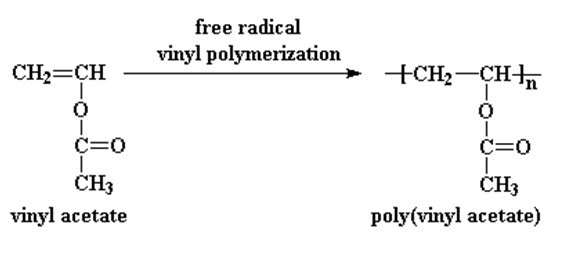
Figure 2. polymerization of vinyl acetate to form polyvinyl acetate (PVAc
The polymerization of vinyl acetate to form polyvinyl acetate (PVAc) is the most important reaction from an industrial and commercial perspective. This polymerization typically occurs via a free-radical mechanism, initiated by peroxides or azo compounds[1-3]. The polymerization is exothermic and can be carried out in bulk, solution, or emulsion[2, 4].
The kinetics of vinyl acetate polymerization have been extensively studied[3, 5]. The rate of polymerization initially increases until about 50% conversion, then decreases rapidly due to the Trommsdorff effect, also known as autoacceleration [3, 5, 6]. This effect is caused by the increasing viscosity of the reaction medium as the polymer forms, which restricts the diffusion and termination of growing polymer chains. As a result, the concentration of active radicals increases, leading to an acceleration in the rate of polymerization [3, 5, 6].
The activation energy for propagation also increases as the polymerization progresses, again due to diffusion limitations in the increasingly viscous medium[6]. This means that the rate constant for propagation becomes more sensitive to temperature as the reaction proceeds.
Various factors can influence the polymerization kinetics and the properties of the resulting PVAc, such as the choice of initiator, solvent, and reaction conditions [2-5]. For example, the use of chain transfer agents can be used to control the molecular weight of the polymer[2, 4].
- Hydrolysis
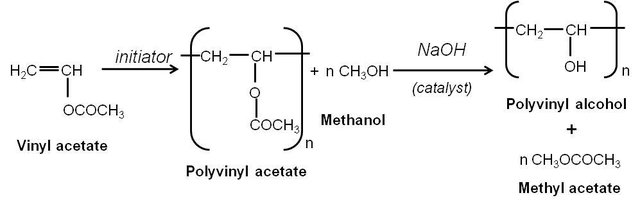
Figure 3. Polymerization of PVAc and hydrolysis of PVAc to PVA[7]
Polyvinyl acetate can be hydrolyzed to form polyvinyl alcohol (PVA), a water-soluble polymer with numerous applications. The hydrolysis is typically carried out under basic conditions using sodium hydroxide or potassium hydroxide [4, 8, 9].
The degree of hydrolysis can be controlled by adjusting the reaction conditions, such as the concentration of base, temperature, and reaction time[8, 9]. Partially hydrolyzed PVAc, known as ethylene-vinyl alcohol copolymer (EVOH), is also commercially important and exhibits properties intermediate between PVAc and PVA[9].
The hydrolysis of PVAc to PVA is an important industrial process, as PVA has many applications in adhesives, textiles, paper coatings, and as a water-soluble packaging material [4, 8, 9].
- Transesterification
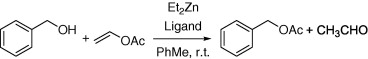
Figure 4. Reaction conditions: BnOH (1 mmol), vinyl acetate (5 mmol), PhMe (2 mL), rt, 1 h[10].
Vinyl acetate can undergo transesterification reactions with alcohols to form other vinyl esters [4, 11-13]. For example, reaction with propanol or butanol would yield vinyl propionate or vinyl butyrate, respectively.
Transesterification is typically catalyzed by acids or bases and involves the exchange of the acetate group with another ester group from an alcohol [11-13]. This reaction provides a route to access a variety of vinyl esters with different properties and applications.
Vinyl esters produced by transesterification can be polymerized in a similar manner to vinyl acetate, yielding polymers with modified properties depending on the ester group[4, 11]. This provides a means to tune the properties of vinyl ester polymers for specific applications.
References
1. A United States Patent 19 11 Patent Number : 5 , 719 , 218 Sarma 45 Date of Patent : Feb. 2017.
2. Zalukaeva, T. and K.G. Kaiser. METHODS FOR MAKING POLYMERS FROM N-VNYLACETAMIDE MONOMER. 2017.
3. Mogilicharla, A., et al., Kriging Surrogate Based Multi-objective Optimization of Bulk Vinyl Acetate Polymerization with Branching. Materials and Manufacturing Processes, 2015. 30: p. 394 - 402.
4. Cordeiro, C.F. and F.P. Petrocelli. Vinyl Acetate Polymers. 2005.
5. Mogilicharla, A., et al., Multi-Objective Optimization of Bulk Vinyl Acetate Polymerization with Branching. Materials and Manufacturing Processes, 2014. 29: p. 210 - 217.
6. Zhou, X., J. Zheng, and X. Zhang, Adsorption of PPL on a high thermal stable DUT-5 and their catalytic activity in a transesterification reaction of cinnamyl alcohol and vinyl acetate. Functional Materials Letters, 2023.
7. Peresin, M., Novel Lignocellulosic Composites. 2011.
8. Li, Y., J. Deng, and J. Zhang, A new-style poly(vinyl alcohol) gel prepared by automatic hydrolysis of poly(vinyl acetate) emulsion. Journal of Applied Polymer Science, 2018.
9. Sangeetha, V., et al., Super toughened renewable poly(lactic acid) based ternary blends system: effect of degree of hydrolysis of ethylene vinyl acetate on impact and thermal properties. RSC Advances, 2016. 6: p. 72681-72691.
10. Shirae, Y., et al., Transesterification of various alcohols with vinyl acetate under mild conditions catalyzed by diethylzinc using N-substituted diethanolamine as a ligand. Tetrahedron Letters, 2005. 46(35): p. 5877-5879.
11. Dhuiège, B., G. Pecastaings, and G. Sèbe, Sustainable Approach for the Direct Functionalization of Cellulose Nanocrystals Dispersed in Water by Transesterification of Vinyl Acetate. ACS Sustainable Chemistry & Engineering, 2018.
12. Pereira, E.C.L., et al., Bronsted acidic ionic liquids: New transesterification agents for the compatibilization of polylactide/ethylene-co-vinyl acetate blends. European Polymer Journal, 2017. 97: p. 104-111.
13. Kumar, M., S.K. Bagchi, and A.S. Sharma, The first vinyl acetate mediated organocatalytic transesterification of phenols: a step towards sustainability. New Journal of Chemistry, 2015. 39: p. 8329-8336.