1. Condensation of polyvinyl alcohol with carbonyl groups (aldehydes, ketones, and quinones) and their applications.
① Development and application of high-viscosity polyvinyl butyral resin (PVB) and PVB film. Polyvinyl butyral interlayers produced using high-viscosity PVB resin exhibit exceptionally strong adhesion, excellent transparency, water resistance, and light resistance. They have long been the preferred material for safety glass interlayers and hold enormous market potential.
② Polyvinyl benzaldehyde is used in the ink-absorbing layer of printing paper. It does not absorb ink well, does not clog printer nozzles, and produces clear images with excellent appearance.
③ Polyvinyl acetals containing strongly hydrophilic groups exhibit rapid water solubility and can reduce chemical surface roughness.
④ Condensation of polyvinyl alcohol with 1,5-dihydroxy-3-pentanone is being used to produce polyvinyl alcohol films that are water-resistant, moisture-resistant, and possess excellent mechanical strength. The product of the condensation reaction of polyvinyl alcohol and p-formyl azophosphonium is used in chromatographic separations and exhibits high sensitivity and distribution coefficients.
⑥ Polyvinyl alcohol is condensed with benzaldehyde containing photosensitive groups to prepare photosensitive resins.
2. Polyvinyl alcohol esterification and applications.
Polyvinyl alcohol can be condensed with acyl chloride or carboxylic acid under acid catalysis to form polyvinyl alcohol esters. Due to the high polarity of the ester bond, polyvinyl alcohol esters exhibit high gloss, hardness, and strong water absorption. Therefore, these polyvinyl alcohols have a wide range of applications.
① The polyvinyl alcohol ester formed by the reaction of polyvinyl alcohol with benzoyl chloride has high surface activity and viscosity.
②) Polyvinyl alcohol borate ester, formed by cross-linking polyvinyl alcohol with boric acid, exhibits excellent elasticity, high hardness, and resistance to the solvents acetic acid and ethane. It is prepared by mixing 1000 parts acetonitrile, 1 part polyvinyl alcohol, and 250 parts LiBO2 into a mold, and drying. Its hardness is 89.5.
③ Blocked polyvinyl acetate is used as a polymer dispersion stabilizer.
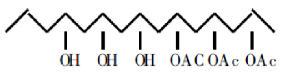
④ Polyvinyl alcohol reacts with chloroacetic acid to form polyvinyl alcohol chloroacetate, a highly effective photosensitive resin.
⑤ Drug molecules with carboxyl groups can achieve sustained release through esterification with PVA. In recent years, PVA has emerged as a promising long-lasting drug. Drugs can be attached to PVA molecular chains or encapsulated within PVA films, then administered orally or implanted in the affected tissue, where they are slowly released to achieve therapeutic effects. This approach offers advantages such as long-lasting efficacy and minimal side effects.
3. Modification by Addition to Form Amide Bonds -NHCO-
① Polyvinyl alcohol is added to isocyanates to produce polyvinyl alcohol polyurethanes. Films made from polyvinyl alcohol polyurethanes exhibit excellent anti-fogging properties and high transparency, with mechanical strength reaching 18 x103 Pa and tear elongation reaching 110%.
② The reaction product of polyvinyl alcohol and diphenylmethyl diisocyanate is used to prepare antiseptic adhesives. The product of the reaction of polyvinyl alcohol and diphenylmethyl diisocyanate is mixed with glycerol ether, benzoate, and a small amount of fragrance to produce an adhesive with antiseptic properties. ③ The reaction product of polyvinyl alcohol and HMPI is used to prepare heat-resistant coatings. The coating produced by the reaction of polyvinyl alcohol and HMPI is heat-resistant, with excellent oil and water resistance and stability. A film of the emulsion prepared by the reaction product of polyvinyl alcohol and HMPI, initiated by diisobutyltin, with vinyl acetate and 2-aminothiophenol, was immersed in toluene at 90°C for 1 hour and lost 3% weight.
3. Modification by Addition to Form Amide Bonds -NHCO-
① Polyvinyl alcohol polyurethane is prepared by addition reaction of polyvinyl alcohol with isocyanate. The film produced by polyvinyl alcohol polyurethane exhibits excellent anti-fog properties and high transparency, a mechanical strength of 18 x103 Pa, and a tear elongation of 110%.
② The reaction product of polyvinyl alcohol and diphenylmethyl diisocyanate is used to prepare an anti-corrosion adhesive. The product of the reaction of polyvinyl alcohol and diphenylmethyl diisocyanate is mixed with glycerol ether, benzoate, and a small amount of fragrance to produce an adhesive with anti-corrosion properties.
③ The reaction product of polyvinyl alcohol and HMPI is used to prepare heat-resistant coatings. The coating prepared by the reaction of polyvinyl alcohol and HMPI is heat-resistant, oil-resistant, water-resistant, and stable. The emulsion prepared by the reaction of polyvinyl alcohol and HMPI with diisobutyltin as the initiator, vinyl acetate, and 2-aminothiophenol was immersed in toluene at 90°C for 1 hour and lost 3% of its weight.
4. Modification through Other Reactions
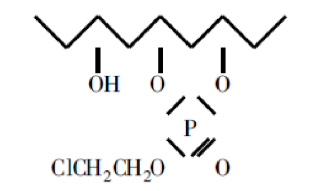
① Formation of P-O bonds. Polyvinyl alcohol is cross-linked with O-(2-chloroethyl)phosphinoyl to form an ester polymer with excellent fire retardant properties.
② Reaction with urea and its derivatives. Polyvinyl alcohol reacts with urea and its derivatives to form modified polyvinyl alcohol polymers. Under UV light, they can undergo photopolymerization to form a paint film. Factors influencing the photocrosslinking reaction include the frequency of the irradiated light, the degree of polymerization of the PVA, and the type of urea derivative.